In a groundbreaking study that could revolutionize medical therapies, scientists from Osaka University in Japan, in collaboration with Monash University in Australia, have unlocked the astonishing potential of regulatory T cells (Tregs) to heal damaged tissues and bones. While these immune cells have often been overlooked, new research shows that Tregs possess remarkable regenerative capabilities, offering hope for innovative treatments in wound healing, bone repair, and possibly even chronic diseases.
Tregs: The Unsung Heroes of the Immune System
Tregs, a specialized subset of immune cells, were previously known primarily for their role in maintaining immune balance and preventing autoimmune responses. However, this new research reveals their broader, untapped potential—they can not only suppress harmful immune reactions but also actively promote tissue regeneration. By influencing other immune cells, such as monocytes and macrophages, Tregs facilitate a healing environment in the body, secreting signaling molecules that encourage tissue repair.
Until now, Tregs’ healing abilities were largely underestimated. The discovery that these cells can switch monocytes and macrophages into a tissue-healing “anti-inflammatory mode” by releasing interleukin molecules (IL-10) is a significant leap forward in understanding how the immune system can be harnessed for therapeutic purposes.
A Regenerative Breakthrough: Healing Bones, Muscles, and Skin
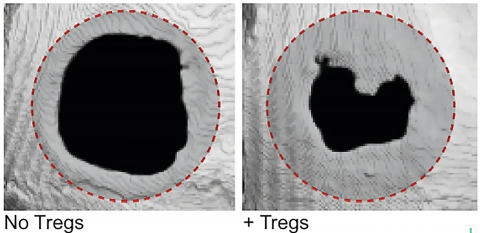
In a series of experiments, the scientists tested the regenerative power of Tregs using a fibrin hydrogel, a protein involved in natural wound healing, to deliver the cells directly into damaged tissues in mice. The results were nothing short of remarkable. Not only did the mice experience accelerated skin wound healing, but they also saw an increase in bone density and muscle fiber size. This was observed even in severe injuries, suggesting that Tregs could be a game-changer in treating hard-to-heal wounds and fractures.
The ability of Tregs to regenerate bone and muscle volume is especially promising for medical treatments aimed at aging populations, where bone density loss and muscle degeneration are common concerns. These findings could also extend to sports medicine, offering athletes new options for faster recovery from injuries.
Changing the Immune Response: From Inflammation to Healing
One of the most intriguing aspects of the study is how Tregs interact with other immune cells to create an environment conducive to healing. When tissues are damaged, the body’s typical immune response involves inflammation, which, while important for fighting infections, can sometimes impede healing if it becomes chronic. Tregs, however, change this dynamic by signaling monocytes and macrophages to focus on repair and regeneration rather than inflammation.
This transformation occurs through the secretion of IL-10, a potent anti-inflammatory cytokine. By encouraging macrophages to adopt a healing role, the research demonstrates how Tregs can essentially reprogram the immune response in a way that accelerates the repair of damaged tissues. This finding could pave the way for treatments that not only heal injuries faster but also reduce the harmful effects of chronic inflammation, a key factor in many degenerative diseases.
The Future of Treg-Based Therapies: A New Hope for Regenerative Medicine
The implications of this research are vast. If Tregs can be harnessed in humans as effectively as they were in these animal models, they could open up new avenues for regenerative medicine. From improving recovery times after surgeries to treating degenerative diseases like osteoporosis or muscular dystrophy, Treg-based therapies could redefine how we approach healing and tissue repair.
One potential application lies in organ transplants, where controlling immune responses is critical. By using Tregs to regulate inflammation and promote healing, transplant patients might experience better outcomes with reduced risk of organ rejection. Furthermore, Treg therapies could also prove valuable in treating autoimmune diseases where tissue damage occurs as a result of the body attacking its own cells.
Moreover, this research offers hope for patients suffering from chronic wounds, such as those with diabetes or other conditions that impair natural healing processes. Current treatments for these types of injuries are often limited and sometimes ineffective. But with Treg-based therapies, patients could see a more robust and reliable healing process, minimizing complications and improving quality of life.
Challenges and Next Steps
While the potential is clear, there are still hurdles to overcome before Treg-based therapies become a clinical reality. One of the major challenges will be scaling this technology for use in humans. Additionally, more research is needed to understand the long-term effects of manipulating Treg activity and ensuring that this approach is safe across a wide range of medical conditions.
Scientists will also need to investigate how to best deliver Tregs to targeted tissues in humans and determine the optimal doses for treatment. Another consideration will be identifying which types of injuries or diseases are most suitable for Treg therapy and developing personalized treatment plans that maximize the benefits of these immune cells.
Tregs at the Forefront of Medical Innovation
The discovery that Tregs can regenerate tissues and bones represents a major leap forward in medical science. By reprogramming the immune system’s response to injury, these cells could drastically improve recovery outcomes for patients across a range of medical fields. From sports injuries to degenerative diseases and wound healing, the potential applications of Tregs are vast and inspiring.
As research continues, we may soon witness the rise of Treg-based regenerative therapies that could transform the landscape of modern medicine, offering new hope to patients around the world. With the power to heal “almost anything,” these once-overlooked immune cells might just be the key to unlocking the future of healthcare.
